`=>` The octet rule, though useful, is not universal.
`=>` It is quite useful for understanding the structures of most of the organic compounds and it applies mainly to the second period elements of the periodic table.
`=>` There are three types of exceptions to the octet rule :
`color{green}("The Incomplete Octet of the Central Atom :")`
● In some compounds, the number of electrons surrounding the central atom is less than eight.
● This is especially the case with elements having less than four valence electrons. Examples are `LiCl, BeH_2` and `BCl_3`.
`Li : Cl \ \ \ \ \ \ \ H : Be : H \ \ \ \ \ \ \ Cl : overset( overset(Cl)( . .))B : Cl`
● `Li`, `Be` and `B` have `1`, `2` and `3` valence electrons only.
● Some other such compounds are `AlCl_3` and `BF_3`
`color{green}("Odd-electron Molecules :")`
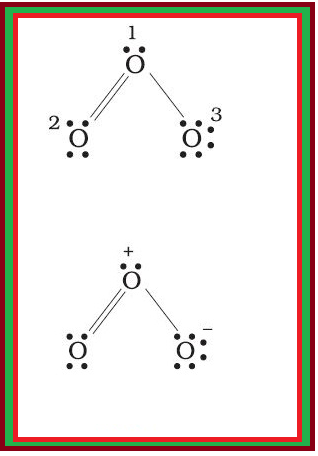
● In molecules with an odd number of electrons like nitric oxide, `NO` and nitrogen dioxide, `NO_2`, the octet rule is not satisfied for all the atoms.
`underset(.)overset(. .)N = underset(. .) overset(. .)O \ \ \ \ \ underset(. .) overset(. .)O= overset(.+)N - underset(. .) overset( . . -)O :`
`color{green}("The Expanded Octet :" )`
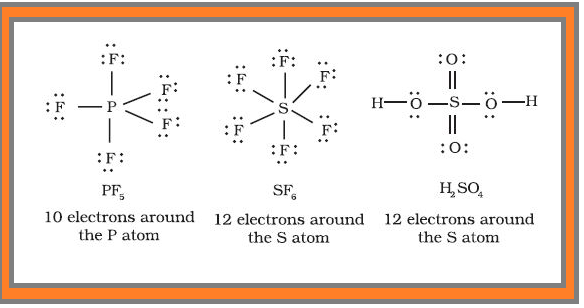
● Elements in and beyond the third period of the periodic table have, apart from `3s` and `3p` orbitals, `3d` orbitals also available for bonding.
● In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Obviously the octet rule does not apply in such cases.
● Some of the examples of such compounds are : `PF_5`, `SF_6`, `H_2SO_4` and a number of coordination compounds.
`color{red}("Note :")` Sulphur also forms many compounds in which the octet rule is obeyed. In sulphur dichloride, the `S` atom has an octet of electrons around it.
`: underset(. .) overset(. .)(Cl) - underset(. .) overset(. .)S - underset(. .) overset(. .)(Cl):` or `: underset(. .) overset(. .)(Cl) : underset(. .)overset(. .)S : underset(. .)overset(. .)(Cl) :`
`=>` The octet rule, though useful, is not universal.
`=>` It is quite useful for understanding the structures of most of the organic compounds and it applies mainly to the second period elements of the periodic table.
`=>` There are three types of exceptions to the octet rule :
`color{green}("The Incomplete Octet of the Central Atom :")`
● In some compounds, the number of electrons surrounding the central atom is less than eight.
● This is especially the case with elements having less than four valence electrons. Examples are `LiCl, BeH_2` and `BCl_3`.
`Li : Cl \ \ \ \ \ \ \ H : Be : H \ \ \ \ \ \ \ Cl : overset( overset(Cl)( . .))B : Cl`
● `Li`, `Be` and `B` have `1`, `2` and `3` valence electrons only.
● Some other such compounds are `AlCl_3` and `BF_3`
`color{green}("Odd-electron Molecules :")`
● In molecules with an odd number of electrons like nitric oxide, `NO` and nitrogen dioxide, `NO_2`, the octet rule is not satisfied for all the atoms.
`underset(.)overset(. .)N = underset(. .) overset(. .)O \ \ \ \ \ underset(. .) overset(. .)O= overset(.+)N - underset(. .) overset( . . -)O :`
`color{green}("The Expanded Octet :" )`
● Elements in and beyond the third period of the periodic table have, apart from `3s` and `3p` orbitals, `3d` orbitals also available for bonding.
● In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Obviously the octet rule does not apply in such cases.
● Some of the examples of such compounds are : `PF_5`, `SF_6`, `H_2SO_4` and a number of coordination compounds.
`color{red}("Note :")` Sulphur also forms many compounds in which the octet rule is obeyed. In sulphur dichloride, the `S` atom has an octet of electrons around it.
`: underset(. .) overset(. .)(Cl) - underset(. .) overset(. .)S - underset(. .) overset(. .)(Cl):` or `: underset(. .) overset(. .)(Cl) : underset(. .)overset(. .)S : underset(. .)overset(. .)(Cl) :`